9th November 2020 – With the pandemic rampaging across the planet for a second wave (and in some countries for a third) it seems that an effective vaccine offers the best hope to the world . Here we examine some of the issues that will lead to this in record time but we caution against the danger of too much haste.
A Blog by Dr David J Flavell PhD FRCPath & Dr Sopsamorn (Bee) Flavell BSc PhD
Scientific Directors of Leukaemia Busters
The Great Vaccine Hope
9th November 2020
Worldwide there are more than one hundred vaccines in development for COVID-19 and on each of these hangs the hope that at least one (and very possibly more than one) will prove effective in defeating the COVID-19 pandemic that is wreaking so much havoc and damage on humankind.
You may well ask the question why are there so many different vaccines in development when they are all designed to do the same job of inducing immunity to the SARS-CoV-2 coronavirus? As the old idiom says “there’s more than one way to cook an egg” and the same is true for many things, including the way in which different types of vaccines are designed to achieve the same end result.
The greater the number of different approaches used the greater is the probability that at least one (or more) will work effectively, so it does make good sense in this health emergency.
We did very briefly cover some of the different types of vaccines in our blog “Vaccinating the Herd” back on the 7th April at a time when COVID-19 vaccines were still in their infancy with many still being only at the research laboratory stage.
Normally vaccine development from conception to roll out takes between 10 to 15 years so the rapid development we have seen over the past six months where there are now several vaccines in phase III clinical trials really is unprecedented and has provided a pointer towards how things might be done more efficiently in future. COVID-19 has already taught us many important lessons this being just another one.
Four Steps to Success
So what’s next? There are four important main steps in the development of a protective vaccine.
- STEP 1 – is to firstly design a vaccine on one of the platforms described in our previous blog “COVID-19 Vaccines:A Cautious Promise” based on information provided by scientific evidence that previous experience suggests will lead to a vaccine that will work. That alone can take years, though newly developed laboratory methods in molecular biology have made the job much easier and quicker in recent times. Already this aspect of COVID-19 vaccine development has been completed for several different vaccines.
- STEP 2 – is to determine if the new vaccine is able to induce immunity in animal models of the infectious disease. Such laboratory-based studies can take many months and cannot be fast tracked because results are limited by the speed with which the animals under study develop an immune response, usually within a time frame of 4 – 8 weeks but sometimes longer. If this step shows that immunity is induced by the vaccine then development can move on to step 3. This step has also been completed for the various COVID-19 vaccines and the results in many (but not all) cases have been published.
- STEP 3 – is then to manufacture sufficient quantities of the clinical grade vaccine to the highest standards of purity known as “cGMP” in readiness to conduct clinical trials in humans. This is expensive, time consuming and potentially tricky step to get the manufacturing conditions required just right. Get it even slightly wrong and this can throw back the whole process by months or more. Major steps have been taken towards contracting several different vaccine manufacturers to manufacture some of the various vaccines in large quantities ready to roll out should the vaccine pass the next big subsequent step of safety and effectiveness in clinical trials.
- STEP 4 – The next enormous step is the design and implementation of clinical trials involving tens of thousands of people who receive the vaccine to firmly establish its safety and additionally and importantly its effectiveness. There’s more than one clinical trial involved here for each new vaccine. In humans the trials are usually split into three different phases I, II and III.
Vaccine Clinical Trial Phases
The phase I trial is what’s termed a dose finding/safety study that see slowly increasing doses of the vaccine administered to groups of individuals to determine a safe dose to use.
Usually the phase II study that follows is takes the dose determined in the phase I and administers this to a smallish group of individuals to measure their immune response usually by looking for antibodies in the blood of vaccinated individuals directed against the viral “antigen” that the vaccine is designed to induce immunity against.
There are also other important things that are examined such as the presence of types of immune cells called T- and B-lymphocytes that target the virus in the vaccinated individuals blood at various times after vaccination. If this proceeds successfully and there is good evidence that the vaccine has indeed induced a good immune response then the trial progresses to phase II.
The phase II trial confirms that the dose selected in the phase I study is appropriate in a relatively small number of individuals (maybe as few as 50) and if this is successful then progresses to phase III.
Phase III is the big one and involves tens of thousands of individuals who receive the safe dose of vaccine established in the phase I/II study described above. In the case of COVID-19 vaccines this is done as what is termed a “double blind” trial where half the participants receive the vaccine and half receive a placebo. Neither participant nor doctor know which the recipient receives the vaccine or the placebo (hence the name “double blind”) which only becomes obvious after the results and identities of the participants are revealed later.
Double Blind trials are done this way in order to avoid any bias in results and to ensure that everyone, whether a vaccine or placebo recipient are treated in exactly the same way apart from in what they receive. That leads us on to talk about the major bottleneck in the process that of determining how effective the vaccine is at protecting against the infection it is intended for.
Overcoming the Bottleneck
There is one major bottleneck in the rapid vaccine development process and it’s something that has to be absolutely clear cut and dry before any national regulatory authority will approve any vaccine for general use in the human population. You’ll see for yourself after we’ve explained that this makes very good sense and is a step that cannot be skipped.
It’s this. Any vaccine HAS to be shown to be effective at protecting the individual against infection with the disease causing organism. As we briefly mentioned above this is normally achieved by allowing tens of thousands of vaccinated individuals to mingle freely in a society where the infection still exists (either epidemically or endemically) and then measure how many of these vaccinated people actually resist become infected in comparison to placebo treated individuals.
By comparing the vaccinated versus placebo groups this then gives a measure as to the extent to which the vaccine has given protection. This phase in the vaccine development process can by its very nature usually take years to complete, years that we cannot afford to wait for in the current pandemic crisis if we are to avoid a see-saw effect of releases and lockdowns.
There is however a way around this and it’s a way that is ethically controversial in our modern world but is probably the only realistic route open to us if we are to have a vaccine ready for general use within the next 6-12 months. What is needed is something called “challenge” studies conducted in vaccinated individuals.
Put simply this involves deliberately attempting to infect vaccinated individuals with the COVID-19 coronavirus and then observing to see how many of these actually do become infected. To do this in a scientifically correct way you also need a group of placebo treated individuals (termed controls) who you also challenge with a dose of the virus in exactly the same way and then wait and see how many from each of the two groups become infected.
Again comparison of the infection rates between the two groups tells you how much the vaccinated group have been protected. We’re sure that you can see the problem of deliberately infecting placebo treated individuals and the potential danger that this poses for them and this makes it difficult ethically.
Regardless, such challenge studies are now being planned in Britain for the Oxford vaccine but are not likely to take place before January of next year. These however are only planned for a relatively young group of people between 20 to 40 years of age who are at relatively low risk. Ideally challenge studies should also be carried out in a large group older people who have been vaccinated to show that protection is safely afforded to them.
This however poses additional ethical issues because older people are at higher risk of developing serious life-threatening symptoms making such a study difficult if not impossible to do.
High Profile Announcements
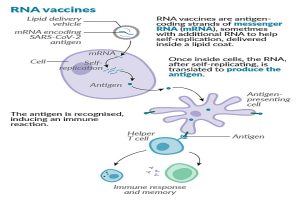
Just today Pfizer announced that its genetic vaccine called BNT162b2 comprised of the viruses genetic material called RNA which encodes the SARS-CoV-2 virus spike protein (the protein on the coronavirus surface that allows it to enter and infect host cells) is 90% effective at safely affording protection to those receiving it. If this vaccine eventually receives regulatory approval it will be the first ever vaccine based on an RNA platform to do so.
This is potentially great news but at this stage we should treat such announcements with caution because the numbers of individuals on which this analysis is based are currently quite small. More study subjects are needed to confirm this and these studies are still ongoing and being extended by Pfizer to achieve the numbers they will need before approval is granted by the various medicines regulatory bodies.
Pfizer chose not to do challenge studies but instead relied on a natural infection rate in individuals receiving the vaccine or the placebo in places where natural infection rates were high. So far of approximately 43,000 individuals in the trial, 94 people have become infected with COVID-19 and the company claims that 90% of vaccinated individuals were protected from infection by their vaccine. These are very preliminary results that have not been reported in full and more work with additional vaccinated and placebo treated individuals are needed to confirm this.
The Pfizer science team are however to be congratulated on the work that has led to this but larger numbers of individuals showing robust protection are needed to confirm these results before everyone becomes too excited that this really is the final answer. Regulatory authorities have however said that under the present emergency conditions they will accept a vaccine that offers a protection rate of 50% or more and it may well be that the Pfizer vaccine will reach or even surpass this target, only time and additional work open to full scientific scrutiny will tell.
Another major advantage of the Pfizer vaccine over others based on living or attenuated viruses is the ease with which it can be manufactured using chemical synthesis methods that are much easier to do at scale. However, the downside is that there are logistical distribution and storage issues with the Pfizer vaccine because in its present form it needs storing at minus 70 degrees celsius (-70C). No doubt work is currently being done to see if this can be improved on by formulating the vaccine is some different way to render it stable at room temperature. That would make the task of storage and distribution so much easier, a particularly important aspect when planning vaccination logistics in developing nations.
In the meanwhile the chase is still on with numerous other vaccine candidates racing towards the finishing line.
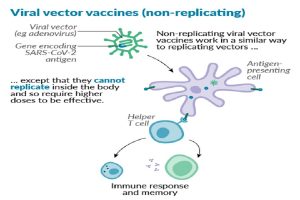
One of Britain’s home grown vaccines from Oxford with the catchy original name ChAdOx1 nCoV-19 also known as AZD1222, is currently in phase III trials around the world and unlike the Pfizer vaccine is based on a genetically engineered “living” chimpanzee virus and is therefore more difficult and more expensive to manufacture in very large quantities.
That said, all the experimental work in animal models and vaccine recipients points to the Oxford vaccine being highly effective at inducing an immune response, though it remains to be seen whether these responses translate into true protection or to just reducing the severity of the COVID-19 illness.
To speed up the process of demonstrating the effectiveness of the Oxford vaccine, plans are now afoot to conduct challenge studies in groups of 20 to 40 year old vaccinated and placebo treated volunteers in January of next year allowing them to arrive at the answer that much more quickly.
There is an ever growing sense of optimism that a safe and effective vaccine will emerge to save the day but some patience is needed to ensure that the right decisions and the right vaccine(s) is chosen to achieve this. Whoever it is that provides this ultimately will have literally saved the world from a ruinous pandemic and put our lives back onto a normal track.
Beyond that let us not forget the lessons that the COVID-19 pandemic has taught us and be better prepared for the next pandemic due to whatever microorganism next comes along, for this will certainly happen one day as surely as night becomes day.
Disinformation – An Ever Present Danger
There’s also another hidden danger about vaccines for COVID-19 that many of you will have heard about and which threatens to thwart any vaccination programme. It’s the problem of disinformation and nonsense conspiracy theories about vaccines perpetrated by players who wish to disrupt and cause problems for reasons best known to themselves. False and/or misleading information will add fuel to the anti-vaxxers camp and potentially dissuade otherwise sensible people not to take up the vaccine once it becomes available.
This could have disastrous consequences where a minimum vaccination take-up rate is needed to afford “herd protection” to the community for this to be an effective way of bringing the pandemic to an end. This issue threatens to disrupt or even prevent a vaccination programme from being effective so much so that it has been reported today that GCHQ is setting up an anti-cyber-war task force to counter this problem.
Be on your guard against this, check the veracity of the source of any information you receive that suggests anything negative about vaccination for COVID-19. If claims that are being made look and sound ridiculous then probably they are ridiculous. It is after all, in your own, your loved ones and the interests of the whole human race to make this work.

While we are waiting for that much needed vaccine it’s more important than ever that we follow the rules to help mitigate further spread of COVID-19 during that wait. Do not ease up with your social distancing, mask use and hand washing, this being something that we’re just going to have to live with for some time to come, perhaps for another six months, perhaps 12 but we will get there and in the meanwhile it’s up to everyone to do their bit in helping prevent more illness and death. Please do continue to strictly follow the trinity of mitigation rules and there’s every chance that infection rates will be kept under sufficient control to prevent any further lockdowns until that much needed vaccine eventually comes along.
A Free High Spec Washable Face Mask for You!

We have obtained specific funding for a supply of high quality reusable/washable 4-ply face masks that we are offering to people in limited numbers for free. To claim your mask please follow the instructions given at the end of the blog entitled Mask Up!.
Note: None of Leukaemia Busters research funds have been used in the supply of these masks.
To read our other blogs click here.
For an up to date on our COVID-19 blog please follow us on Twitter:


